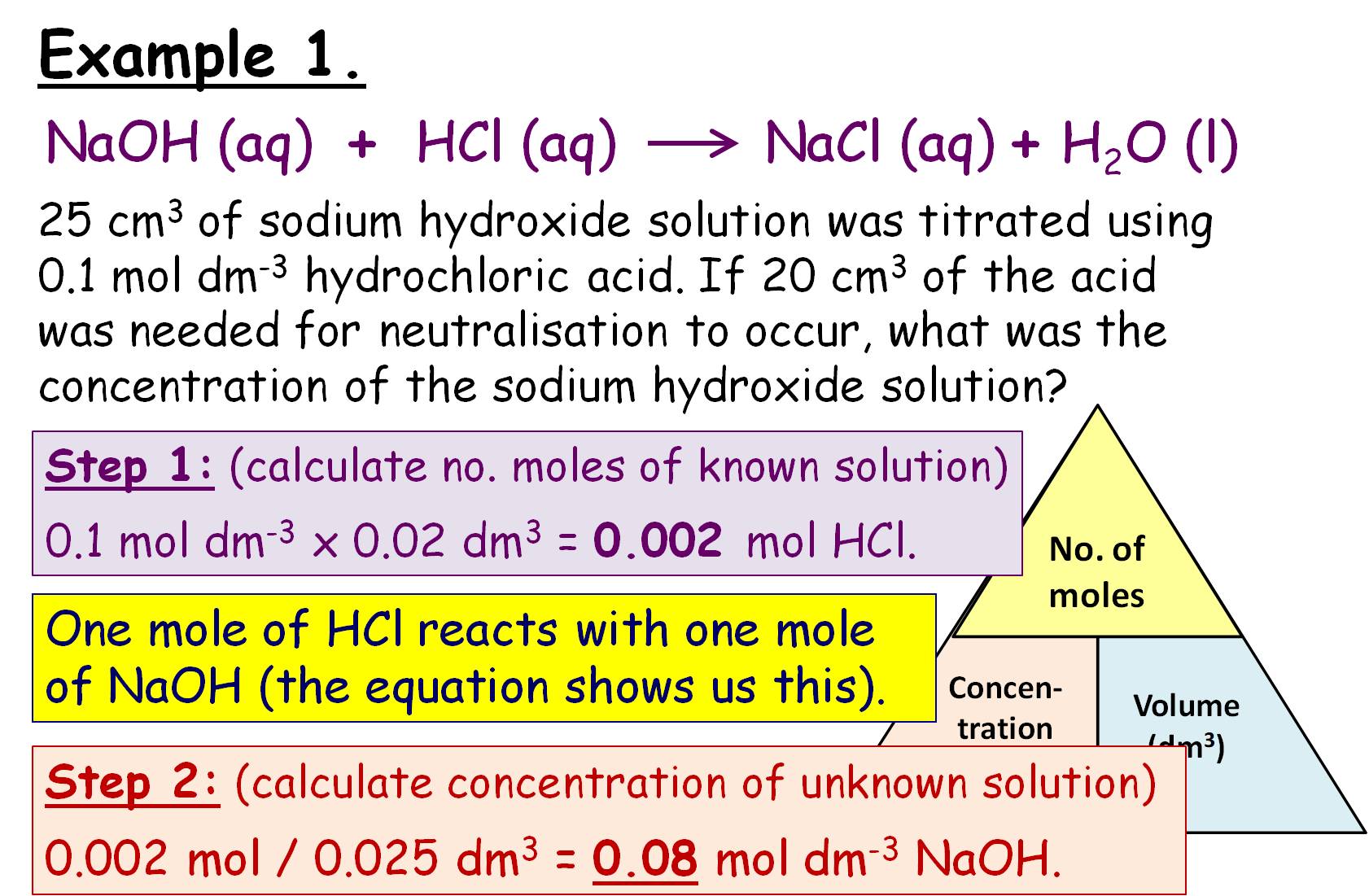
Titration Equations Step By Step . Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. List the known values and plan the problem. the steps in a titration reaction are outlined below. the first step is to figure out what region of the titration curve you are in, and then solve the problem. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. Calculate the amount of sodium hydroxide in moles. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an.
from learningzonelisannin.z14.web.core.windows.net
List the known values and plan the problem. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. the steps in a titration reaction are outlined below. Calculate the amount of sodium hydroxide in moles. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. the first step is to figure out what region of the titration curve you are in, and then solve the problem. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an.
Chemistry Titration Questions And Answers
Titration Equations Step By Step Calculate the amount of sodium hydroxide in moles. the steps in a titration reaction are outlined below. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. the first step is to figure out what region of the titration curve you are in, and then solve the problem. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. Calculate the amount of sodium hydroxide in moles. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. List the known values and plan the problem.
From chem.libretexts.org
Titration of a Strong Acid With A Strong Base Chemistry LibreTexts Titration Equations Step By Step The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. List the known values and plan the problem. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added. Titration Equations Step By Step.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration Equations Step By Step the first step is to figure out what region of the titration curve you are in, and then solve the problem. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. . Titration Equations Step By Step.
From www.science-revision.co.uk
Titrations Titration Equations Step By Step List the known values and plan the problem. Calculate the amount of sodium hydroxide in moles. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. the steps in a titration reaction are outlined below.. Titration Equations Step By Step.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Equations Step By Step The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. the steps in a titration reaction are outlined below. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and. Titration Equations Step By Step.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Equations Step By Step the steps in a titration reaction are outlined below. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. the first step is to figure out what region of the titration curve you are in, and then. Titration Equations Step By Step.
From www.youtube.com
Step by step titration calculation YouTube Titration Equations Step By Step The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. List the known values and plan. Titration Equations Step By Step.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Equations Step By Step Calculate the amount of sodium hydroxide in moles. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. the steps in a titration reaction are outlined below. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. The titration vessel is charged with the solvent and karl fischer reagent, and the. Titration Equations Step By Step.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Titration Equations Step By Step the first step is to figure out what region of the titration curve you are in, and then solve the problem. Calculate the amount of sodium hydroxide in moles. the steps in a titration reaction are outlined below. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. A measured volume. Titration Equations Step By Step.
From missmeyerscience.blogspot.com
Miss Meyer's Science Site Titration Calculations Titration Equations Step By Step List the known values and plan the problem. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. Calculate the amount of sodium hydroxide in moles. the first step is to figure out what region of the titration curve you are in, and then solve the problem. A measured volume of an acidic solution (the analyte) whose concentration. Titration Equations Step By Step.
From slideplayer.com
Titration. ppt download Titration Equations Step By Step the steps in a titration reaction are outlined below. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. Calculate the amount of sodium hydroxide in moles. Volume of sodium hydroxide solution. Titration Equations Step By Step.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Equations Step By Step Calculate the amount of sodium hydroxide in moles. the first step is to figure out what region of the titration curve you are in, and then solve the problem. the steps in a titration reaction are outlined below. Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. For a neutralization reaction with a 1:1 stoichiometric ratio. Titration Equations Step By Step.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Equations Step By Step Calculate the amount of sodium hydroxide in moles. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. List the known values and plan the problem. the first step is to figure out what region of the titration curve you are in, and then solve the problem. Volume of sodium hydroxide solution. Titration Equations Step By Step.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Equations Step By Step Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. List the known values and plan the problem. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. Calculate the amount of sodium hydroxide in moles. The titration vessel is charged with the solvent and karl fischer reagent, and the system is. Titration Equations Step By Step.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Equations Step By Step the steps in a titration reaction are outlined below. Calculate the amount of sodium hydroxide in moles. List the known values and plan the problem. the first step is to figure out what region of the titration curve you are in, and then solve the problem. The titration vessel is charged with the solvent and karl fischer reagent,. Titration Equations Step By Step.
From www.tffn.net
Solving Titration Problems A StepbyStep Guide The Enlightened Mindset Titration Equations Step By Step the first step is to figure out what region of the titration curve you are in, and then solve the problem. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. A. Titration Equations Step By Step.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Equations Step By Step Volume of sodium hydroxide solution = 25.0 ÷ 1,000 = 0.0250. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. Calculate the amount of sodium hydroxide in moles. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. List the known values. Titration Equations Step By Step.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration Equations Step By Step For a neutralization reaction with a 1:1 stoichiometric ratio between the acid and the base, at the. List the known values and plan the problem. A measured volume of an acidic solution (the analyte) whose concentration is unknown is added to an. Calculate the amount of sodium hydroxide in moles. the steps in a titration reaction are outlined below.. Titration Equations Step By Step.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Equations Step By Step the first step is to figure out what region of the titration curve you are in, and then solve the problem. Calculate the amount of sodium hydroxide in moles. The titration vessel is charged with the solvent and karl fischer reagent, and the system is conditioned to ensure stability. For a neutralization reaction with a 1:1 stoichiometric ratio between. Titration Equations Step By Step.